|
◆ 会议时间:2026年11月10-13日
◆ 会议地点:西班牙 马德里
◆ 会议简介:
2026年第21届国际感染病学大会(ICID2026)将于2026年11月10-13日在西班牙马德里举行,会议由国际感染病学会(ISID)组织,预计有超过2000名专业人士与会。
国际感染病学会(ISID)成立于1986年,由国际感染病大会(ICID)和国际感染病与寄生虫病联合会(IFIPD)合并组建,宗旨是宣改善感染性疾病患者的护理,培训指导感染病学和微生物学临床医生和研究人员以及控制世界各地的感染性疾病等;目前ISID在全球155个国家拥有超过80000名会员。ISID及其成员致力于发展伙伴关系,并寻求全球传染病问题的解决方案。未经许可禁止复制摘录转载本站任何内容-国际医学会议网(lingyuint.com).
ICID 2026 – 21st International Congress on Infectious Diseases
时间/Date:
November 10-13, 2026
地点/Venue:
Madrid, Spain
Organized by:
International Society for Infectious Diseases (ISID)
下为上届信息:
ICID 2024 – 20th International Congress on Infectious Diseases
摘要征文投稿:
- All abstracts must be received on or before 31 March 2024.
- Abstract acceptance notifications will be sent by 7 July 2024.
点此提交摘要>>>Submit Abstract>>>
Maximum abstract submissions per person – 5
Themes
Abstracts should be submitted under one of the following themes (click to view sub-themes):
(1) Microbes, pathogenesis and host immunity (2) Antimicrobials (3) Clinical infection and infectious diseases (4) Influenza, COVID-19 and respiratory virus epidemics (5) Healthcare associated Infections (6) Parasitology and vector-borne infections (7) Infections in special populations and settings (8) Fungal Infections (9) Virology (10) One Health (11) Outbreaks, Infectious Disease Surveillance and Epidemiology (12) Innovations in infection detection, prevention and control (13) Social contexts and public health control (14) HIV/AIDS (15) Tuberculosis and Mycobacterial infections (16) Vaccines and Vaccine Development
Abstract Guidelines
General Information
- All abstracts for the Congress must be submitted electronically through the electronic abstract submission system.
- All abstracts must be submitted in English.
- All abstracts must be received on or before 31 March 2024.
- All abstracts have a 450-word limit of text (not including presenter information and title).
- Abstracts must be based on original results that have not or will not be published or presented before the Congress begins on 3 December 2024.
- Submitting authors are required to ensure that all listed co-authors have reviewed the abstract, take responsibility for its content, and agree with being listed as a co-author.
- All potential conflicts of interest for each co-author must be provided.
- Potential conflicts of interest include but are not limited to, commercial interest in the research and financial or in-kind support for the research from entities with a commercial interest in the research.
- Abstract acceptance notifications will be sent by 7 July 2024.
- Only the highest-scoring accepted abstracts will be published as an online supplement to the International Journal of Infectious Diseases (IJID)
Additional Information
- We expect that more than 900 abstracts will be presented during the Congress. Eighty reviewers from more than 30 countries are reviewing and scoring abstracts.
- The abstract review process is blinded, meaning that authors’ names and affiliations are not disclosed to reviewers.
- Each abstract will be reviewed and scored by two reviewers for the quality and originality of the research, the scientific content, and the presentation.
- A notice of acceptance or rejection will be sent to the submitting authors only. It is the responsibility of the submitting author to notify other co-authors of the Committee’s decision.
- Session assignments will be mailed by 31 August 2024, to the address given for correspondence.
- Acceptance of the abstract in the program and assignment to a session are determined solely by the Program Committee based on the abstract’s subject matter and combined reviewer scores and comments.
- Decisions of the Program Committee are final.
- Because of the logistics of assigning abstracts to sessions and the number of abstracts received, requests for particular types of presentations, sessions, days, or times will not be considered by the Program Committee.
- Presenters of accepted abstracts must be registered and attend the Congress in order to have the abstract published in the online supplement to the International Journal of Infectious Diseases (IJID).
- If the registration fee of the presenting author is not received by 31 August 2024 the abstract will not be published in the Final Program.
- By submitting an abstract the author(s) agree to transfer all copyrights to ISID and to allow publication of submitted information on the ISID website, the International Journal of Infectious Diseases (IJID), or other Society related publications.
- Instructions will be sent to the authors whose abstracts are accepted for oral or poster presentations.
- For each abstract accepted for presentation, at least one author must attend the Congress and present the contribution.
Abstract Categories
Abstracts may be accepted into one of three categories:
Oral Presentation – Highest scoring abstracts will be selected for oral presentations.
The presenting author will be required to make a 7-minute PowerPoint presentation that concisely summarizes the research question, methods used, the results, and their significance.
Abstracts accepted for oral presentation will be published as an online supplement to the International Journal of Infectious Diseases.
Poster Presentation – The presenting author will be obliged to present the poster during the moderated poster session in Cape Town to which it has been assigned.
The highest-scoring abstracts accepted for poster presentation will be published as an online supplement to the International Journal of Infectious Diseases (IJID).
Consider the following information according to your type of submission:
a) Research Abstracts
It is recommended that research abstracts follow the format below:
Background/Introduction: A statement of the hypothesis or research question.
Methods & Materials: An explanation of the study design and experimental method and materials used.
Results: A concise summary of the major findings of the experiment or study. Sufficient data must be provided to permit evaluation by the
reviewers and public reading of the abstract. Statements such as “additional information to be presented at the meeting” are not acceptable.
Discussion: where you comment on the results of the study, make comparisons with other studies, discuss whether more research is needed or make recommendations that could be applied in practice.
and
Conclusion: Summary of the overall findings and the importance of the study.
- Abstracts that represent the presentation of investigations of compounds that involve inadequate numbers of study subjects, in vitro studies of non-representative microbiologic strains, or abstracts that lack quantitative or qualitative data will not be accepted.
- Authors are discouraged from submitting multiple abstracts from the same project.
- Each abstract will be judged on its own merits without reference to other submissions.
- Repetitive abstracts will be rejected.
- There is a limit of 5 abstracts you can submit. However, an author may present no more than two abstracts. If more than two submissions are accepted from an author, one of his/her co-authors must present any additional abstracts.
- The presenter of any accepted abstract must be one of the co-authors listed on the original submission.
- Please note that authors may not submit the same research; abstracts containing identical or nearly identical data from the same institution and/or individuals will be rejected.
b) Clinical Case Reports
A Case Report is a brief description of a particular condition that is unusual and also provides new insights into diagnosis or clinical management.
A Case report must make a distinct, novel contribution to the understanding of the etiologic agent, its clinical manifestations, and/or its diagnosis or treatment.
It is recommended that clinical case reports follow the format below:
Background: A statement on the particular condition that is unusual.
Case Description: A description of key features of the case. Sufficient data must be provided to permit evaluation by the reviewers and public reading of the abstract.
Discussion: A concise discussion of the distinct, novel contribution of the case to the understanding of the disease, its clinical manifestations, and/or its diagnosis or treatment.
Conclusion: Summary of the overall findings and the importance of the case report.
Case reports must be authentic, understandable, educational, and clinically interesting to an international audience of healthcare professionals, researchers, and others in all infectious diseases subspecialties. Each case report will be judged on its own merits without reference to other submissions. Repetitive case reports will be rejected.
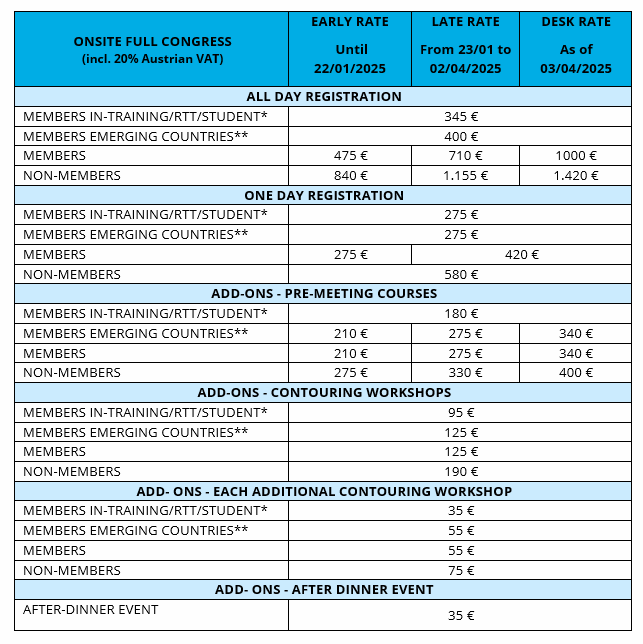
Registration Fees
| Registration Categories |
Registration Fee |
| Full Participant – Early Bird Registration (valid until 2024/05/30) |
$575.00 |
| Full Participant – Regular Registration (valid until 2024/09/30) |
$850.00 |
| Full Participant – Late Registration (from 2024/10/01) |
$950.00 |
| Student / Early Career Researcher |
$425.00 |
| Low and Lower Middle Income Countries (LMICs)* |
$425.00 |
| FIDSSA Member |
$722.50 |
Registration for the Congress includes
- Admission to Plenary sessions, Scientific sessions, Policy Discussions and Meet-the-Expert sessions
- Opportunity to purchase and attend Pre-Congress Workshops
- Earn CME credits by attending the scientific sessions and Pre-Congress Workshops
- Opening ceremony and welcome reception (1 person)
- Access to the Exhibition Hall
- Access to Poster displays
- Delegate kit and Congress materials
|